The National Institute for Pharmaceutical Technology & Education (NIPTE) is a collaboration of academia, industry and other stakeholders using research, outreach and education to improve the way medicines are designed, developed and manufactured to meet the needs of patients in the 21st century. NIPTE leverages scientific and technological advances to bring confidence and reliability for pharmaceutical products to consumers worldwide. During the NIPTE 2018 Research Conference at Long Island University last month, guest speakers talked about the current state of pharmaceutical product development. One of the topics discussed was continuous manufacturing.
Dr. Douglas Hausner, Associate Director Industrial Liaison, Rutgers University, provided an overview of continuous manufacturing. According to Dr. Hausner, continuous manufacturing has been shown to greatly reduce the time and cost of developing new medicines while enabling significant improvements in quality and reliability.
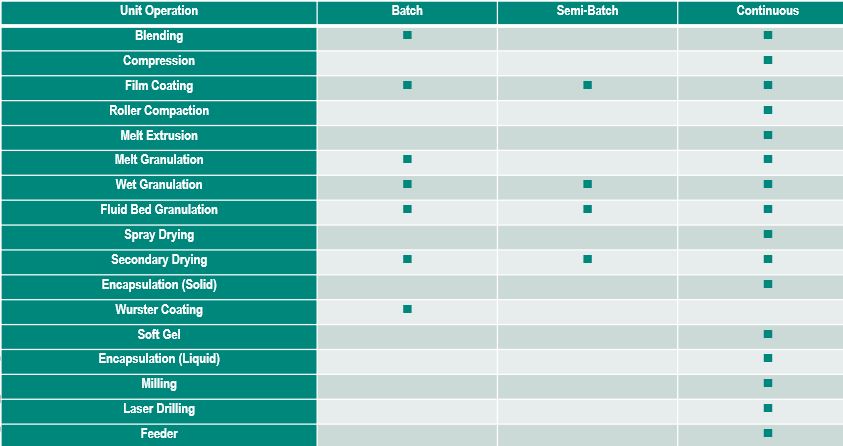
Dr. James DiNunzio, Senior Principal Scientist, Merck, discussed Development Considerations for Continuous Manufacturing. Dr. DiNunzio provided a chart that illustrates the differences between batch and continuous manufacturing.
As you develop your continuous manufacturing R&D strategy, ask yourselves these questions:
- What unit of operation are you using?
- How will you integrate your operations?
- What new equipment will you need?
- Are there new materials required?
- What new control approaches are required?
NIPTE’s 2018 Research Conference did a great job of providing insight into the current state of pharmaceutical development. Specifically, this was true in the area of continuous manufacturing in the pharmaceutical industry. Yes, there are many challenges yet to be discovered but part of the journey is discovering the problem and developing solutions to overcome those problems.
Freund-Vector is interested in learning about your solid dosage continuous manufacturing journey. Why are you considering adopting continuous manufacturing for your tablets and capsules? What are your challenges? When are you expecting to start this journey? We have developed the Granuformer® , which is an innovative system that can perform granulation and drying continuously. The granules are continuously delivered into a newly developed spiral dryer and dried by heated air, and then collected by the cyclone.
