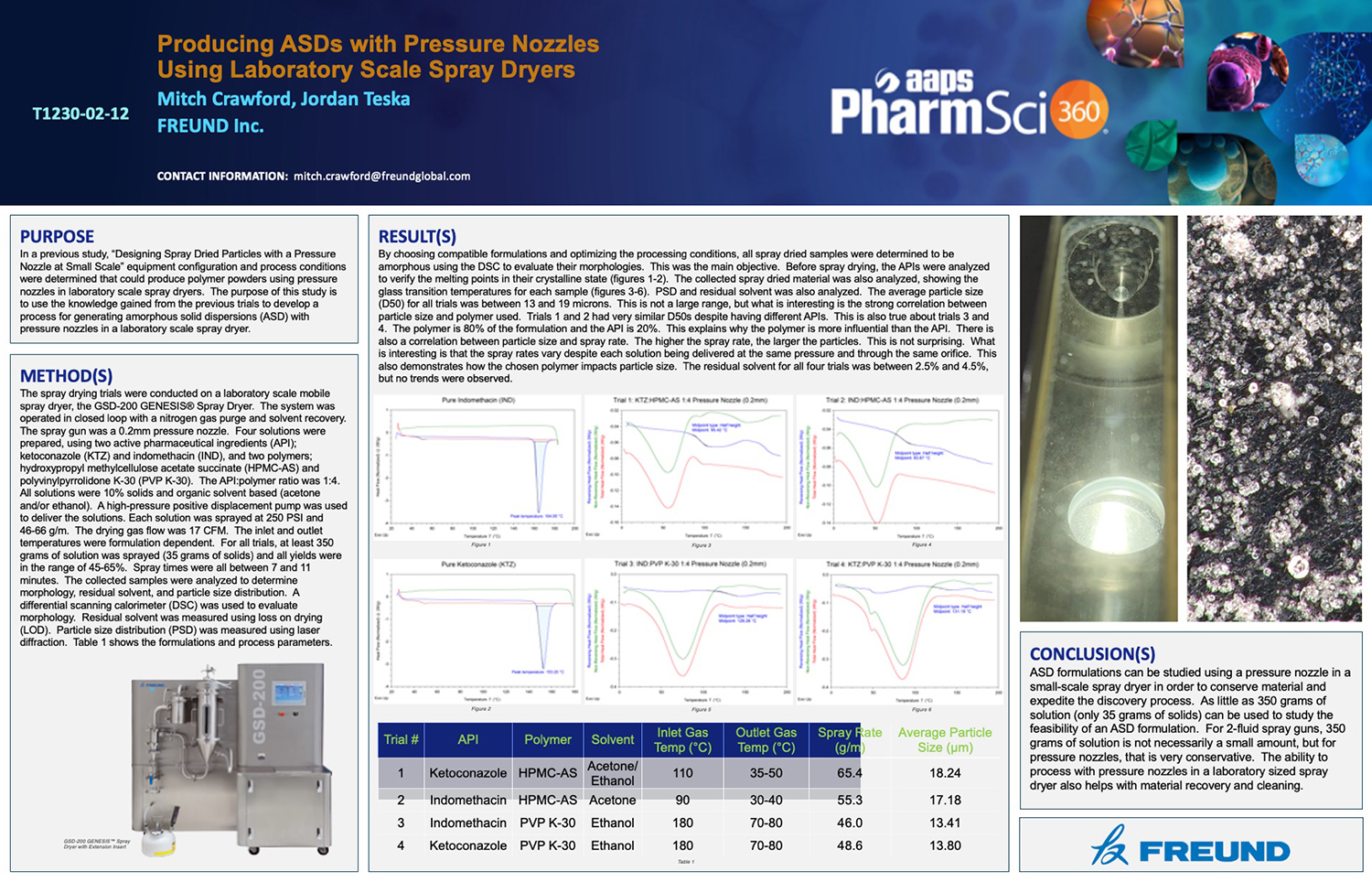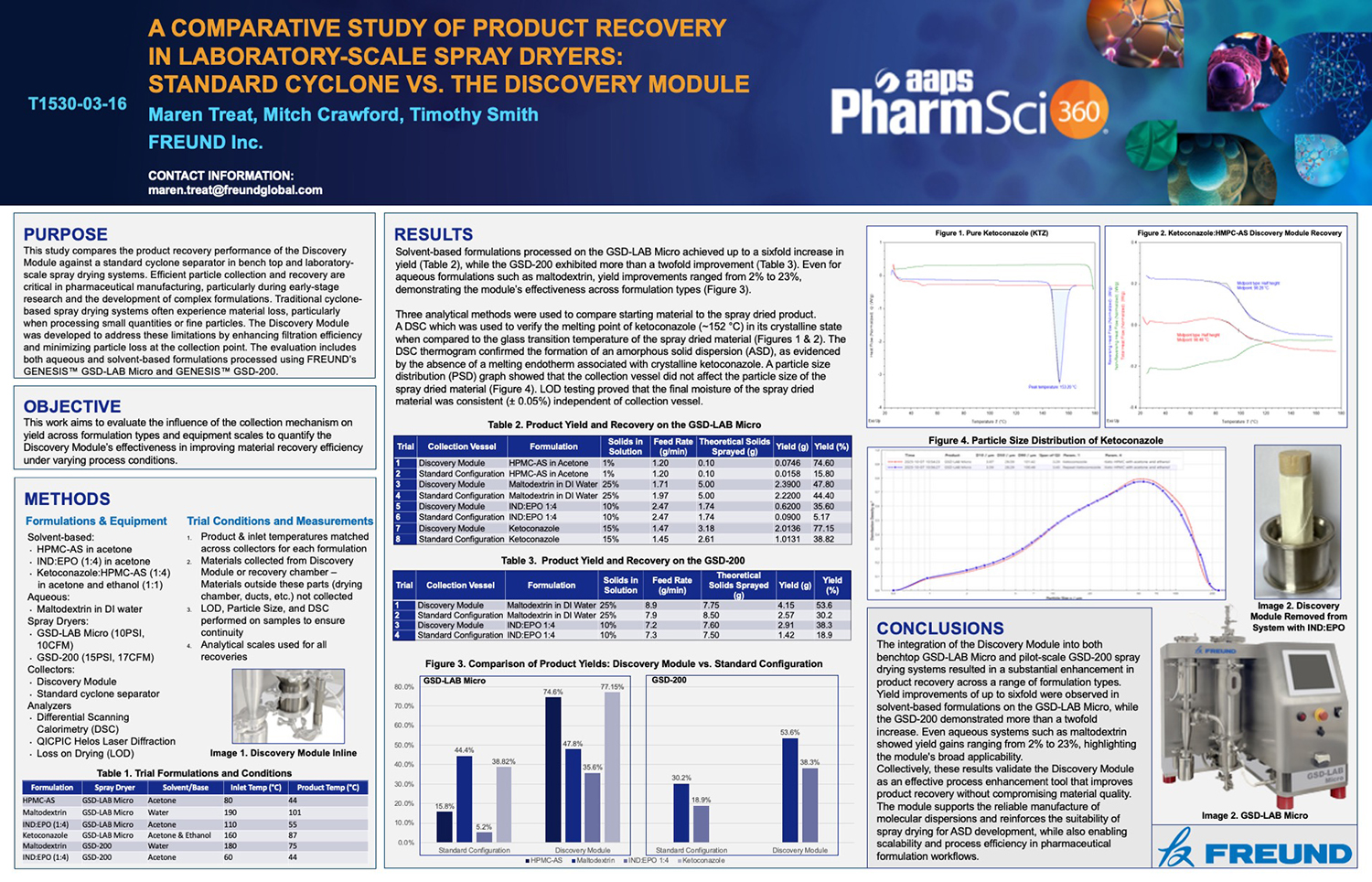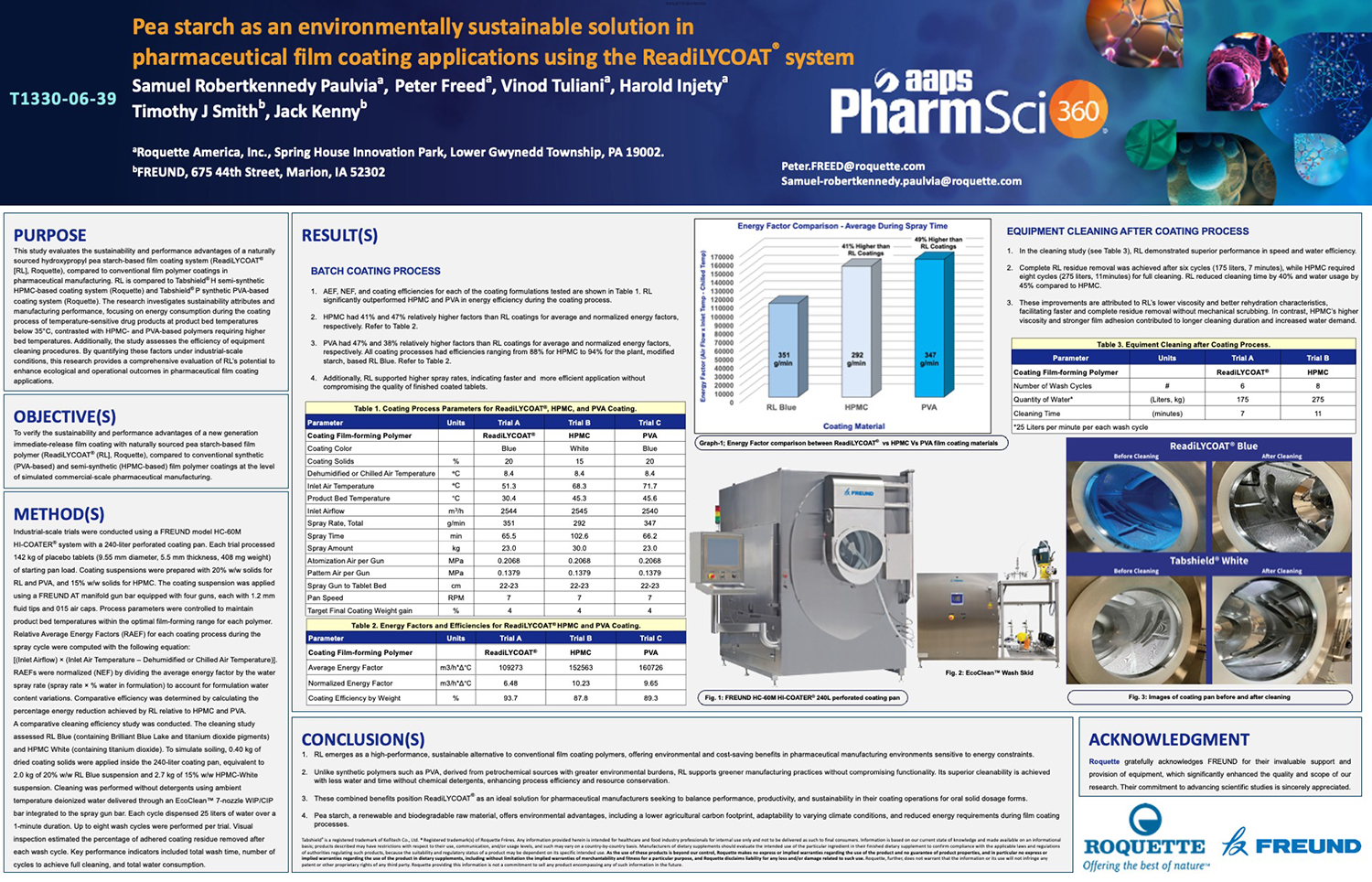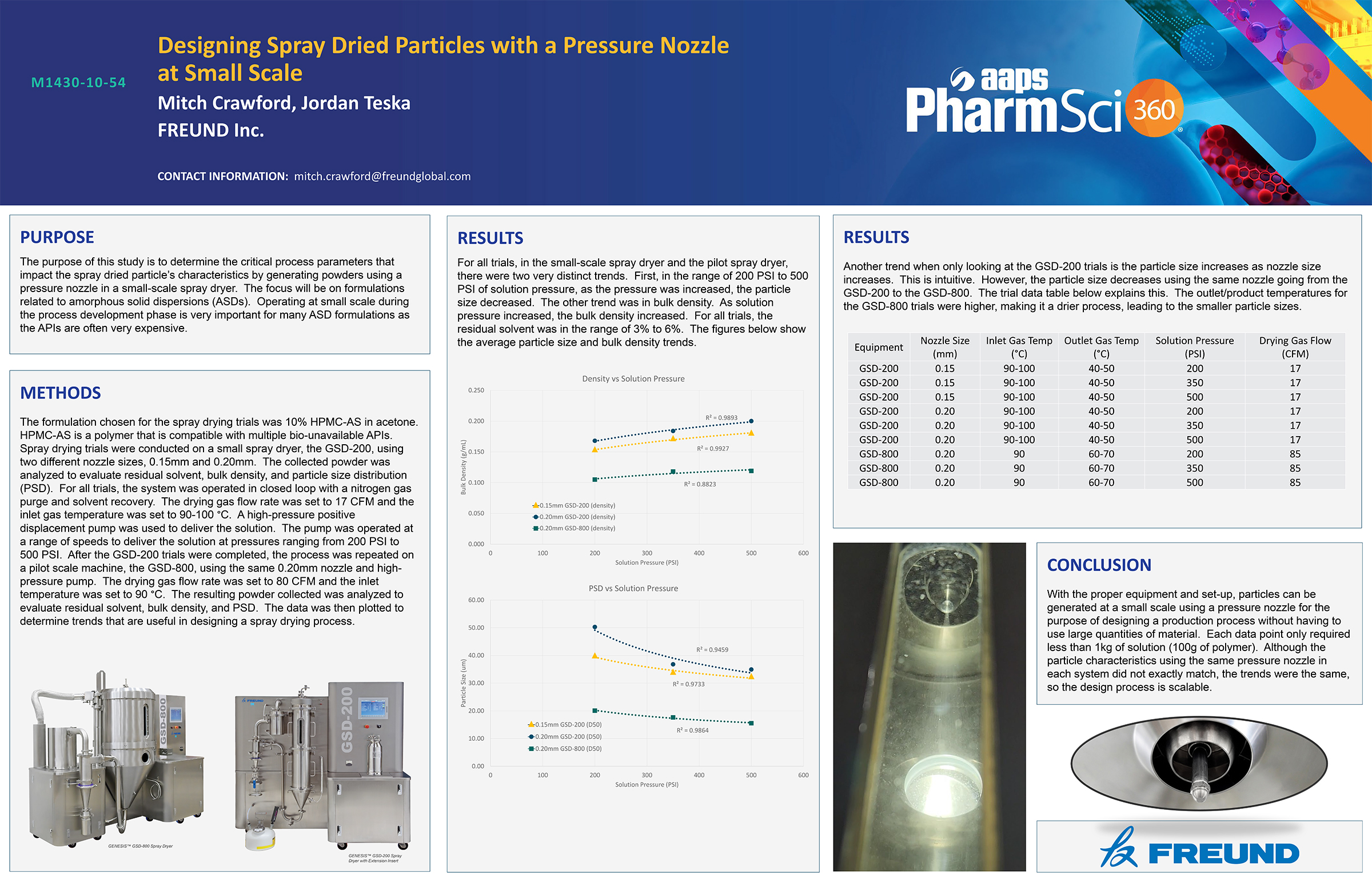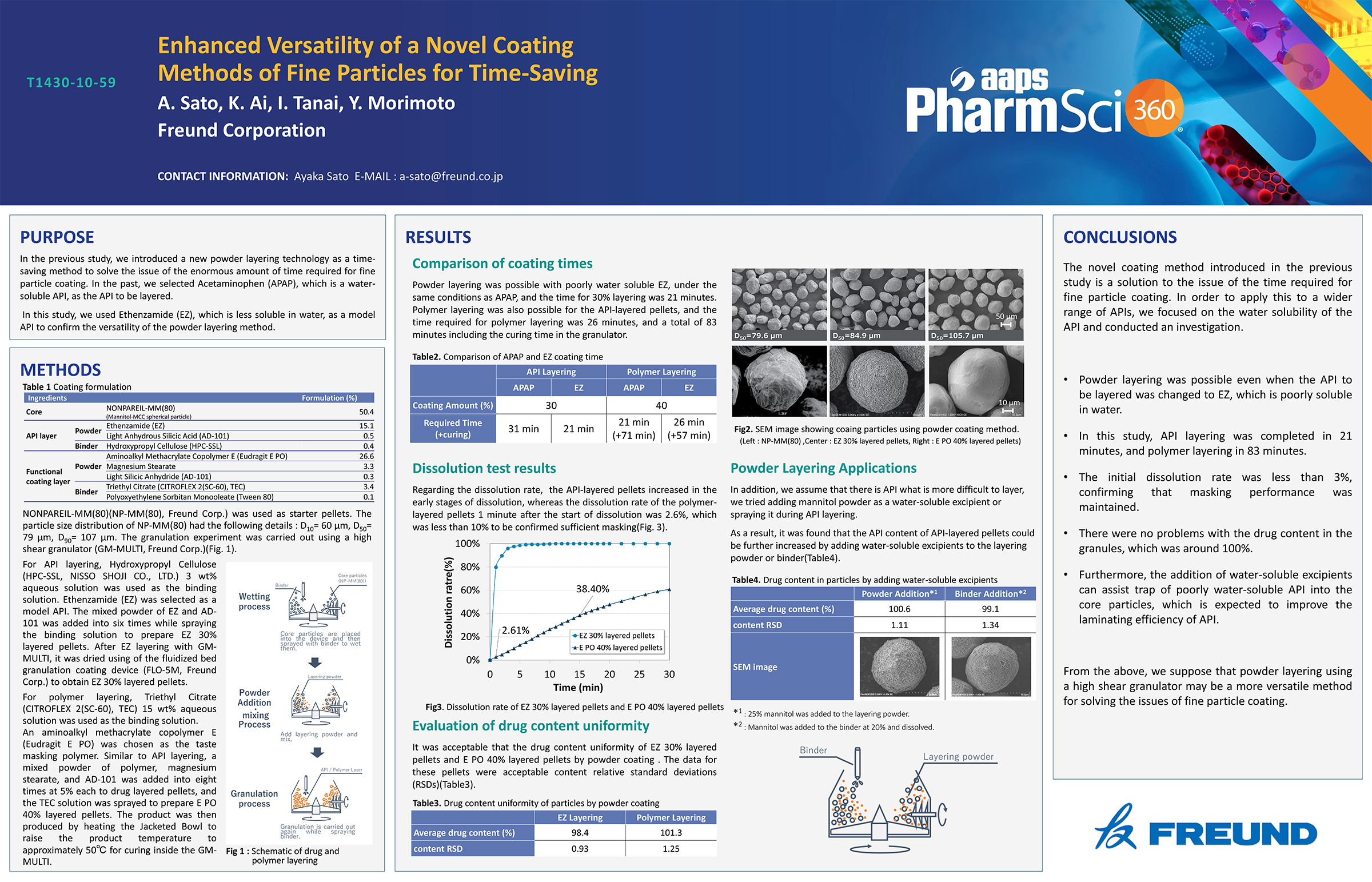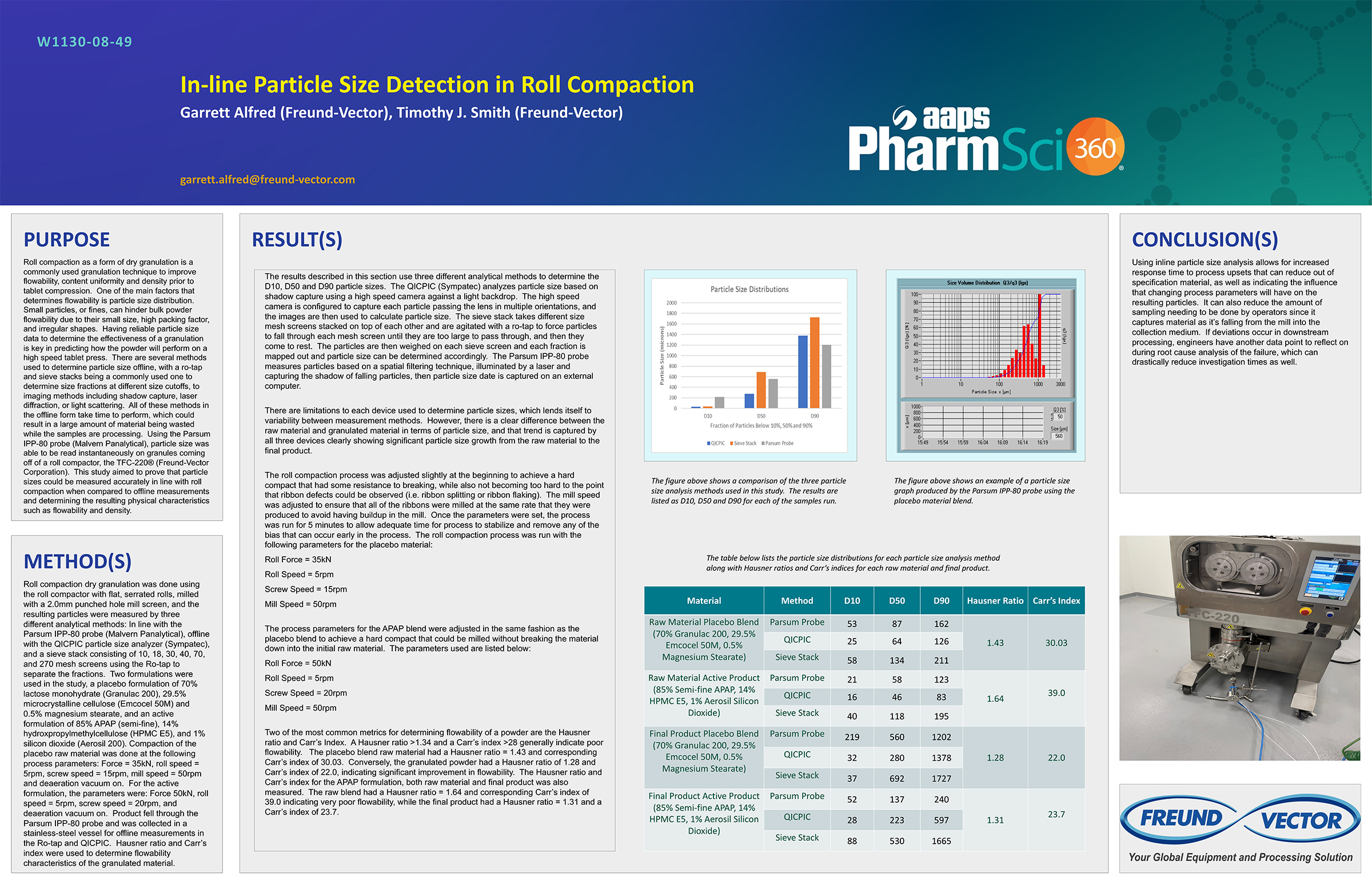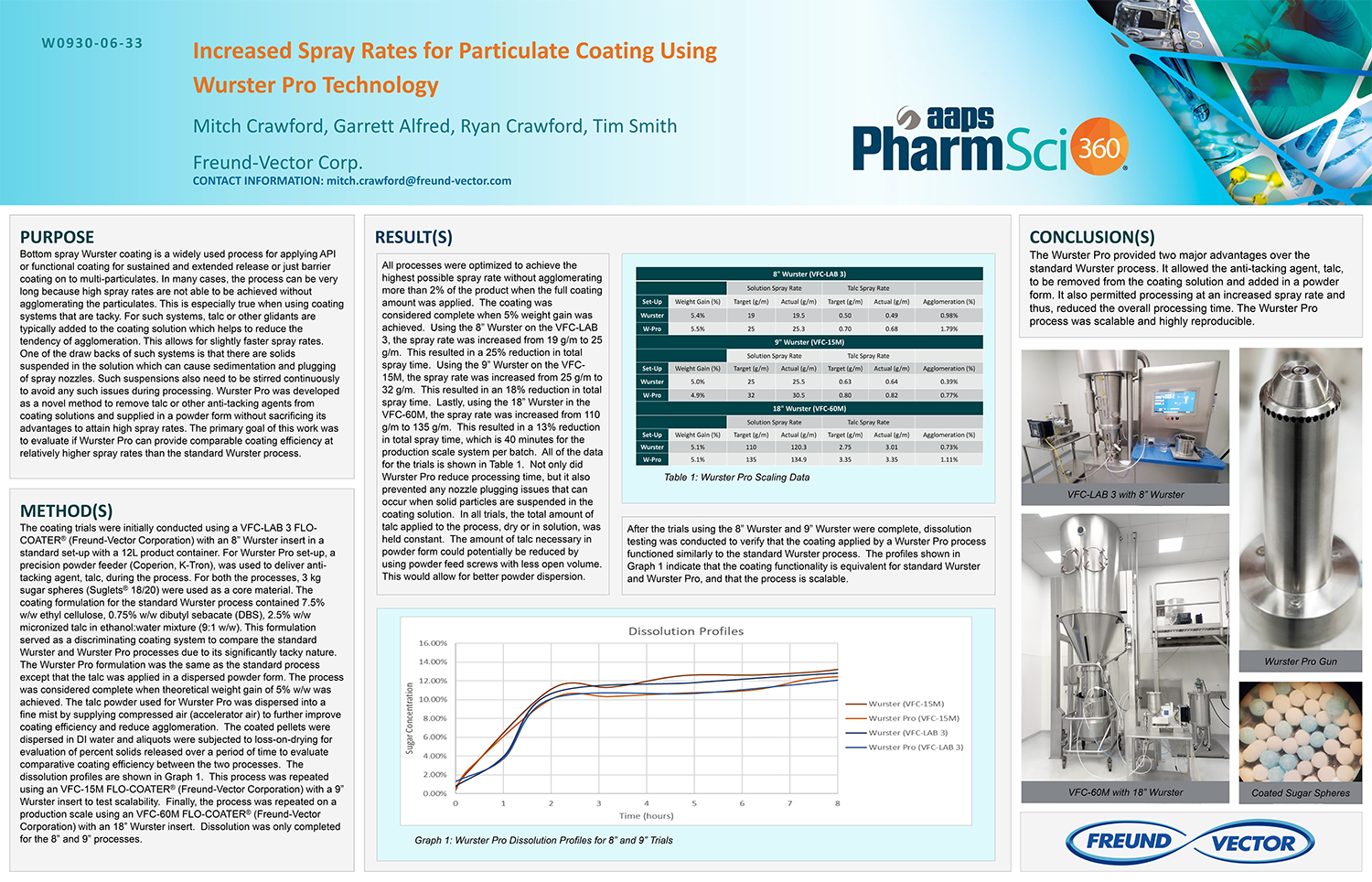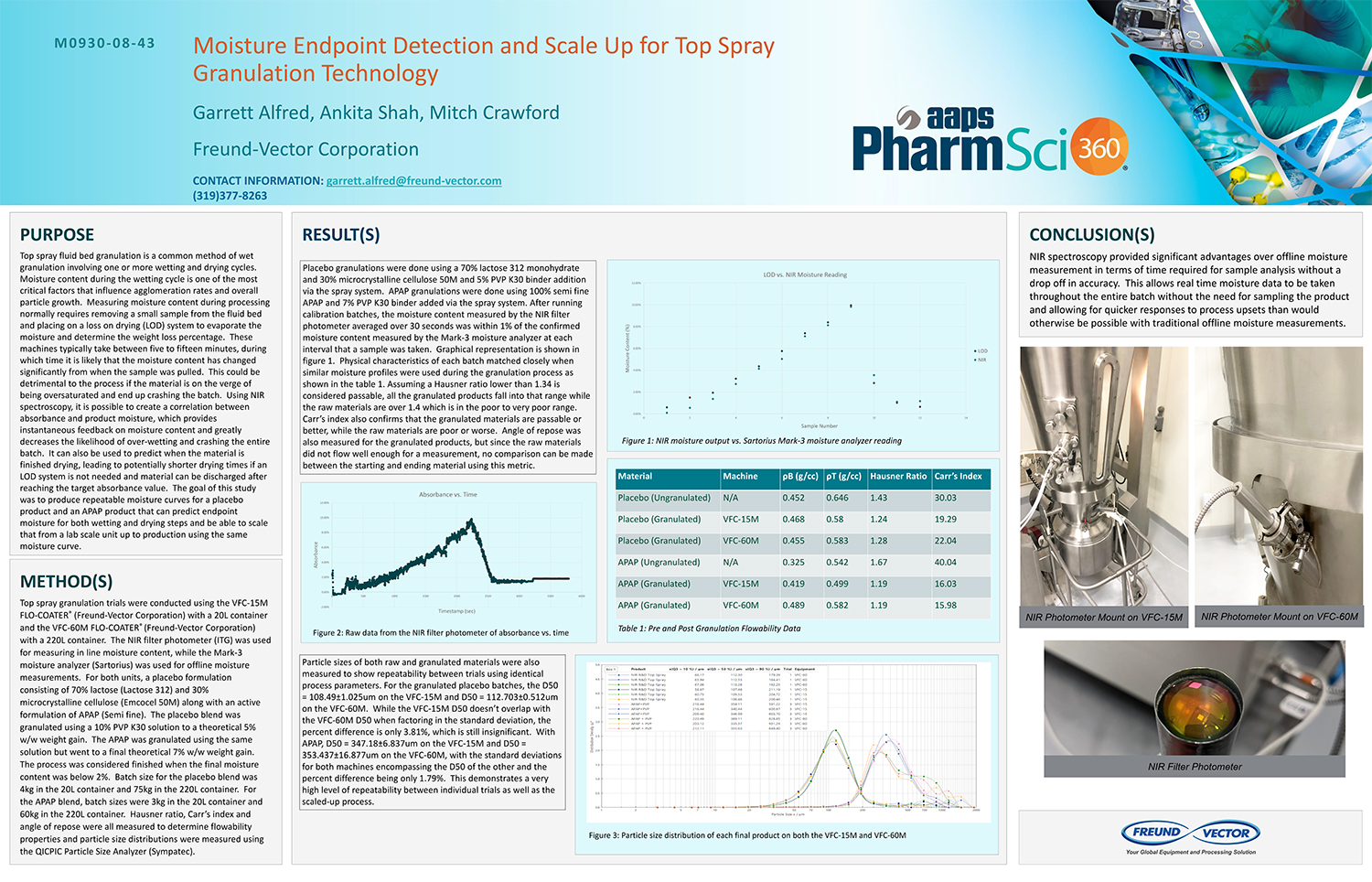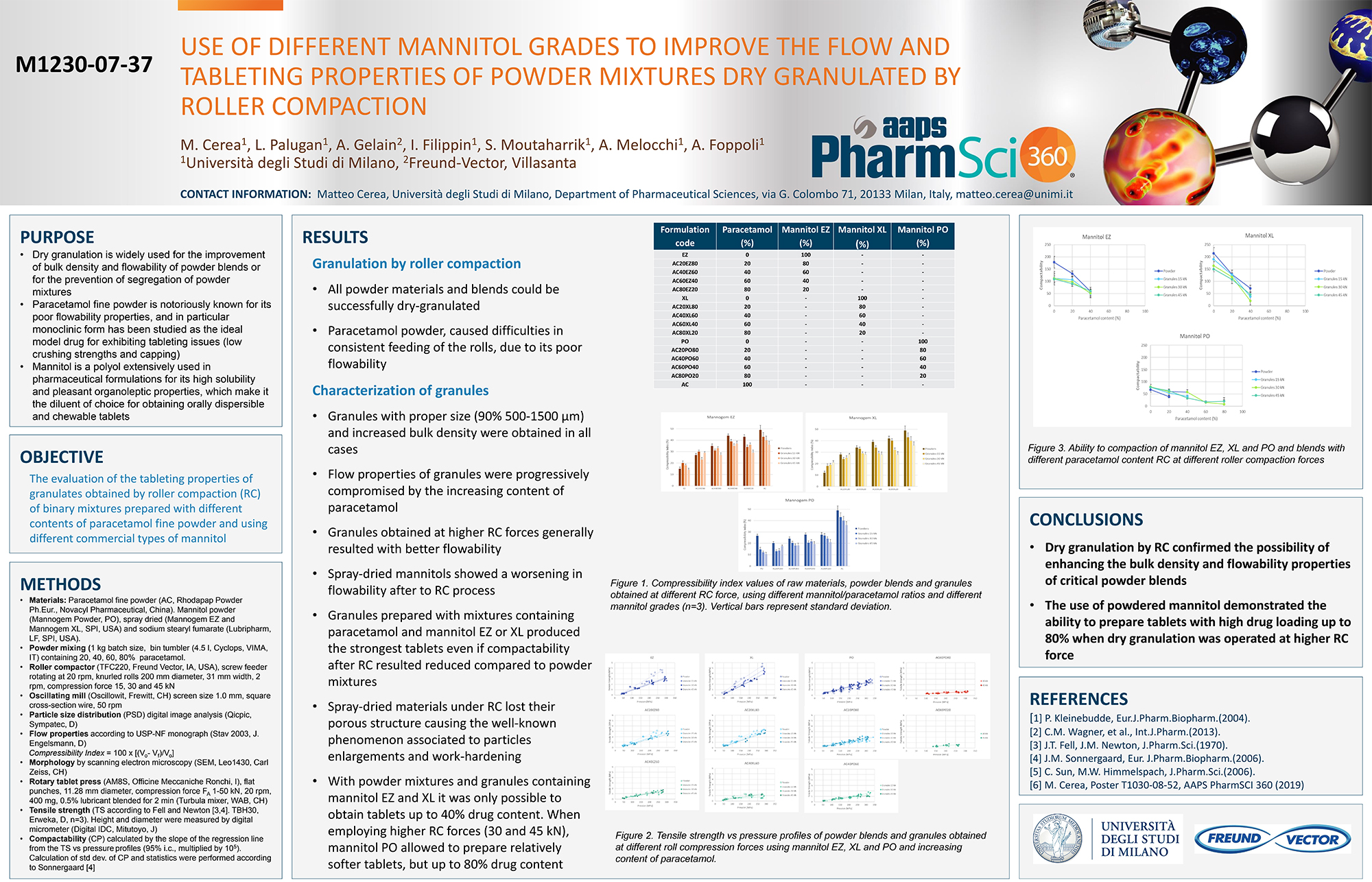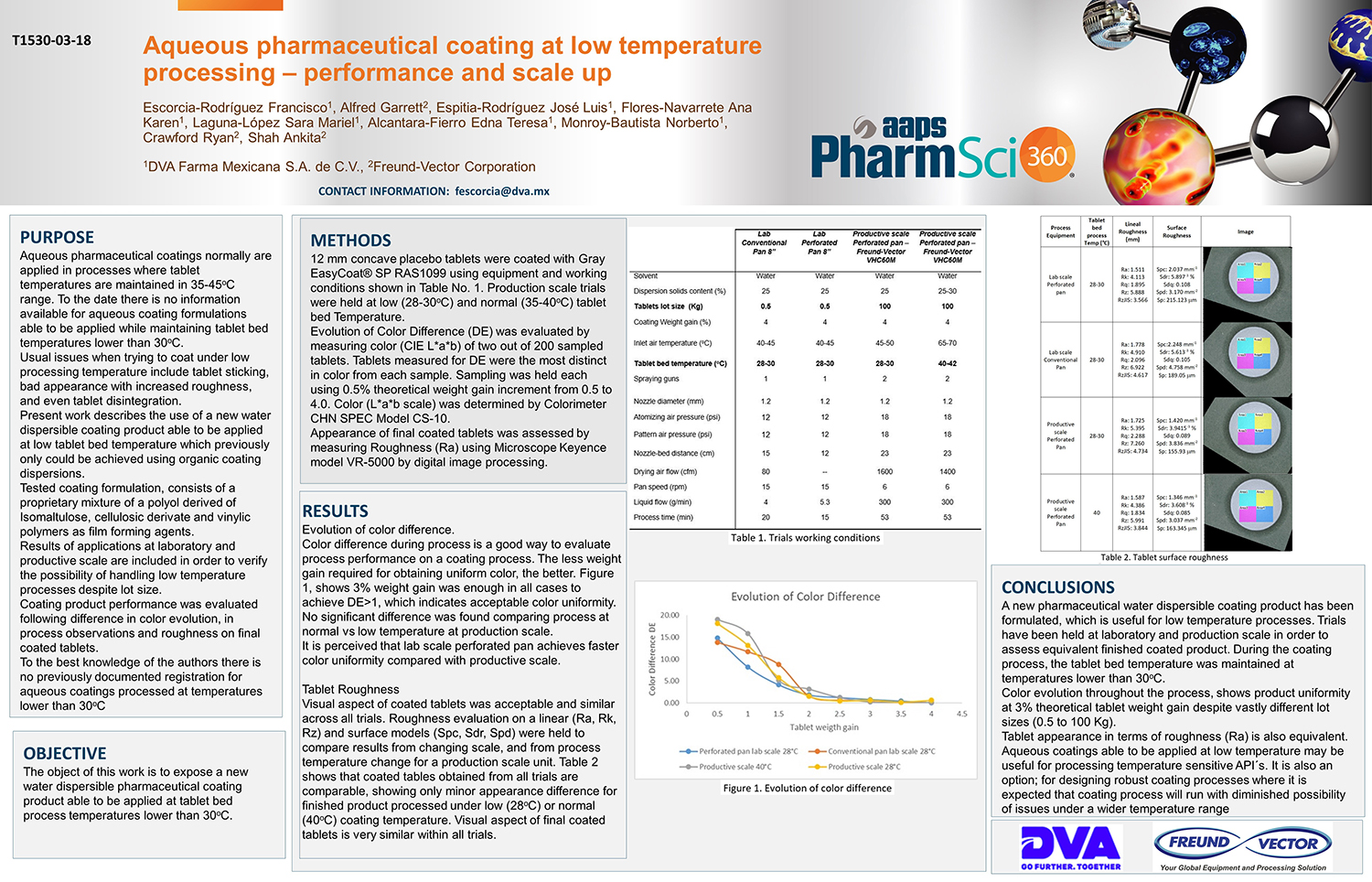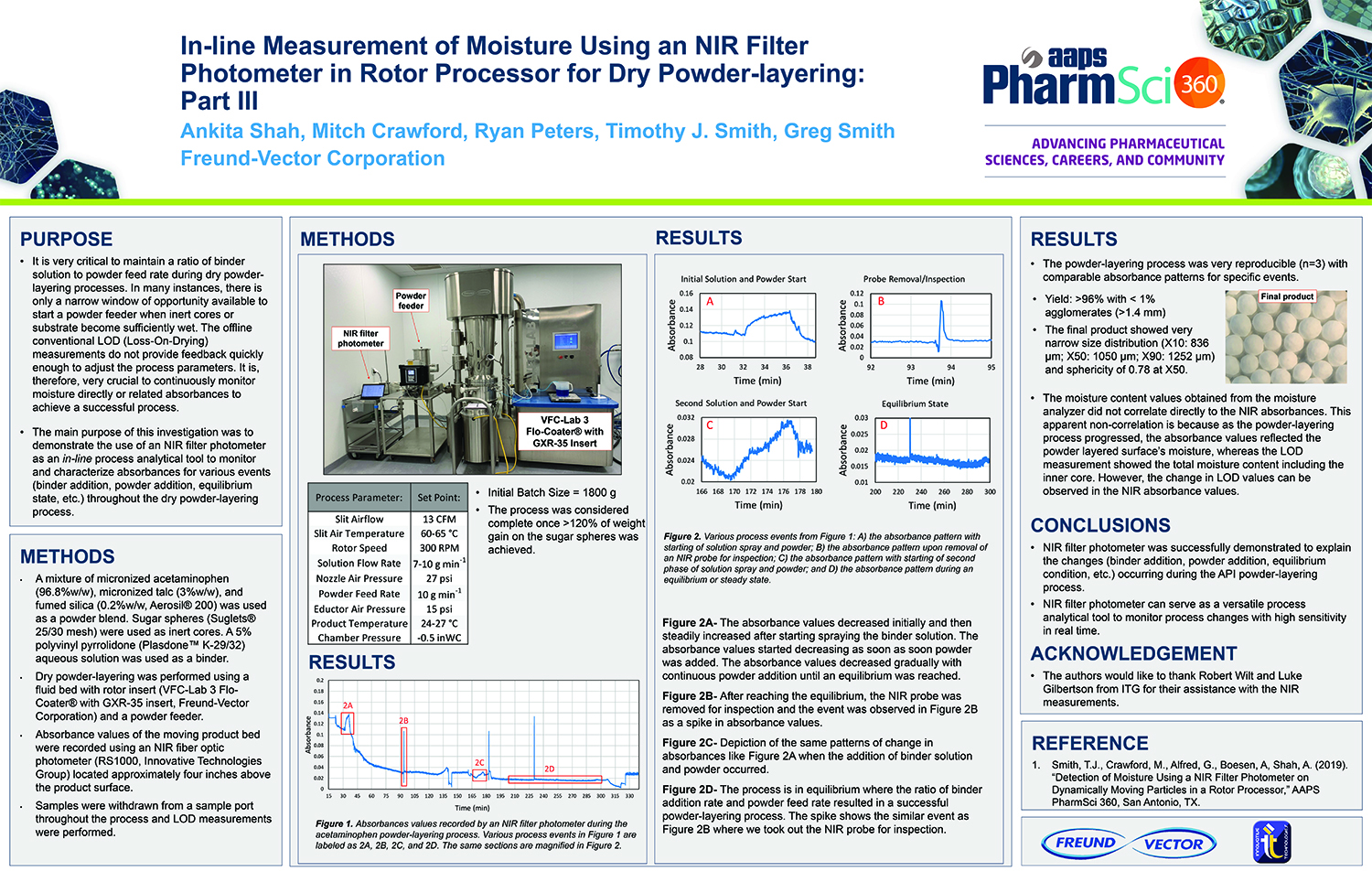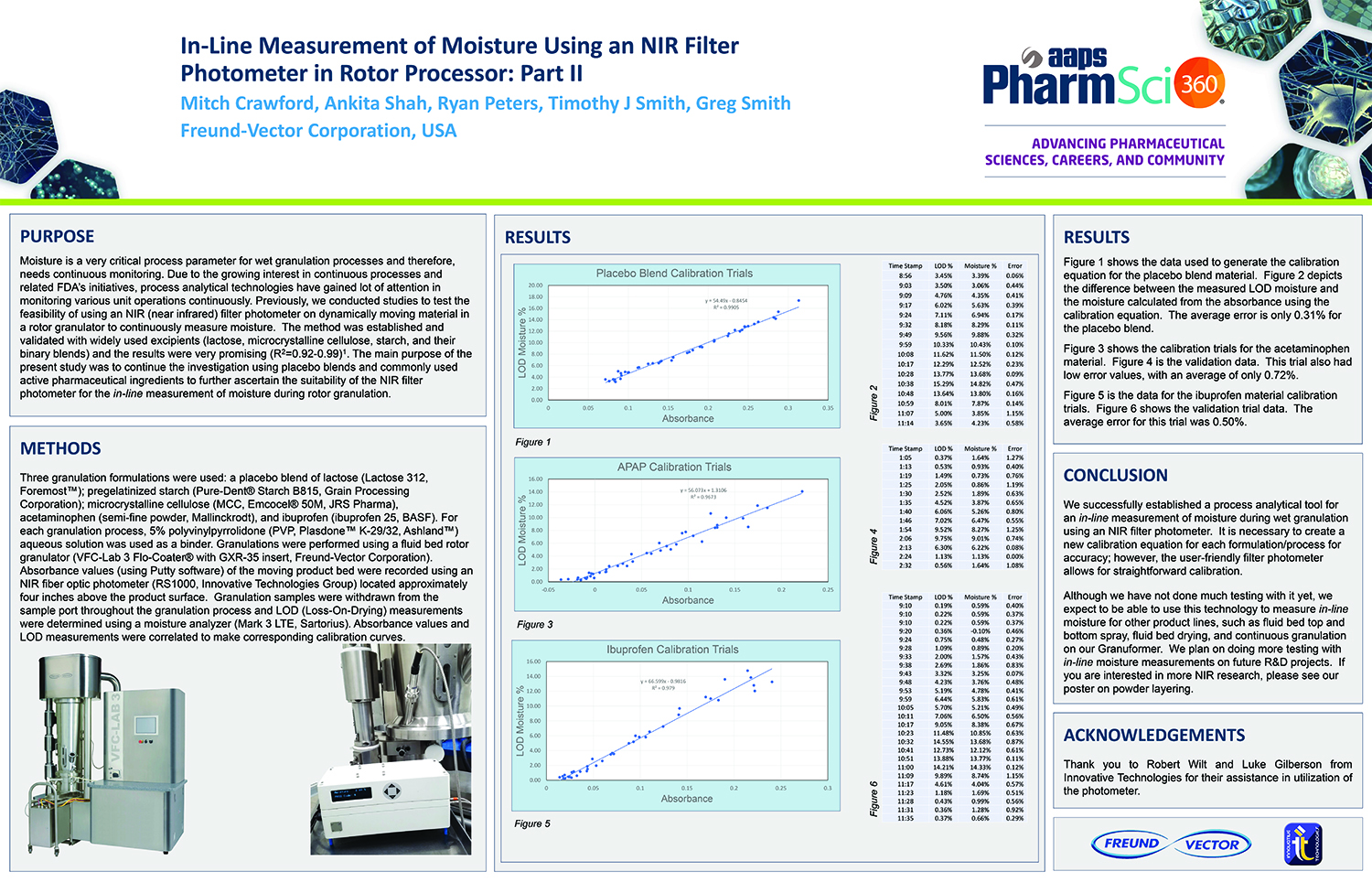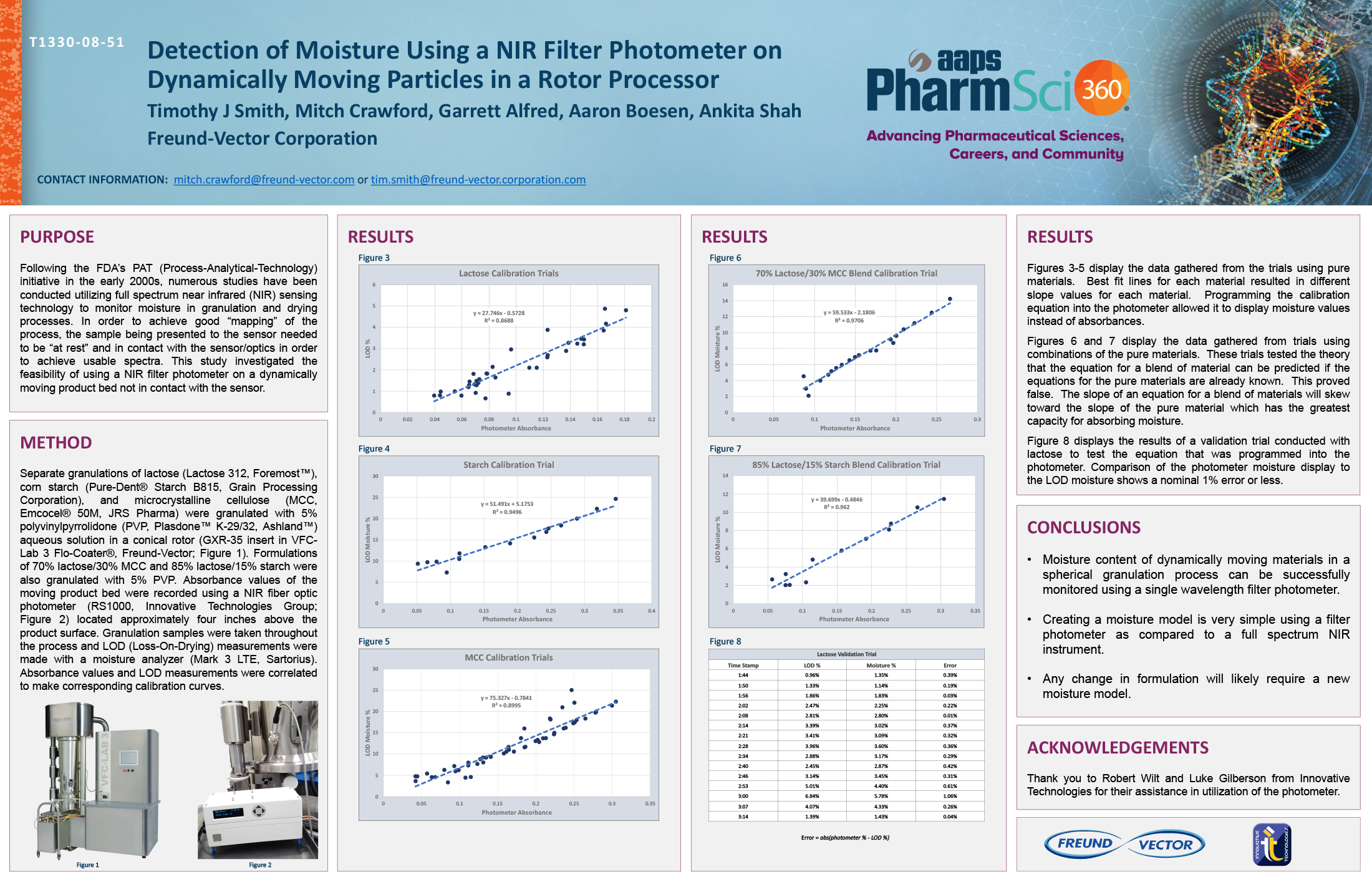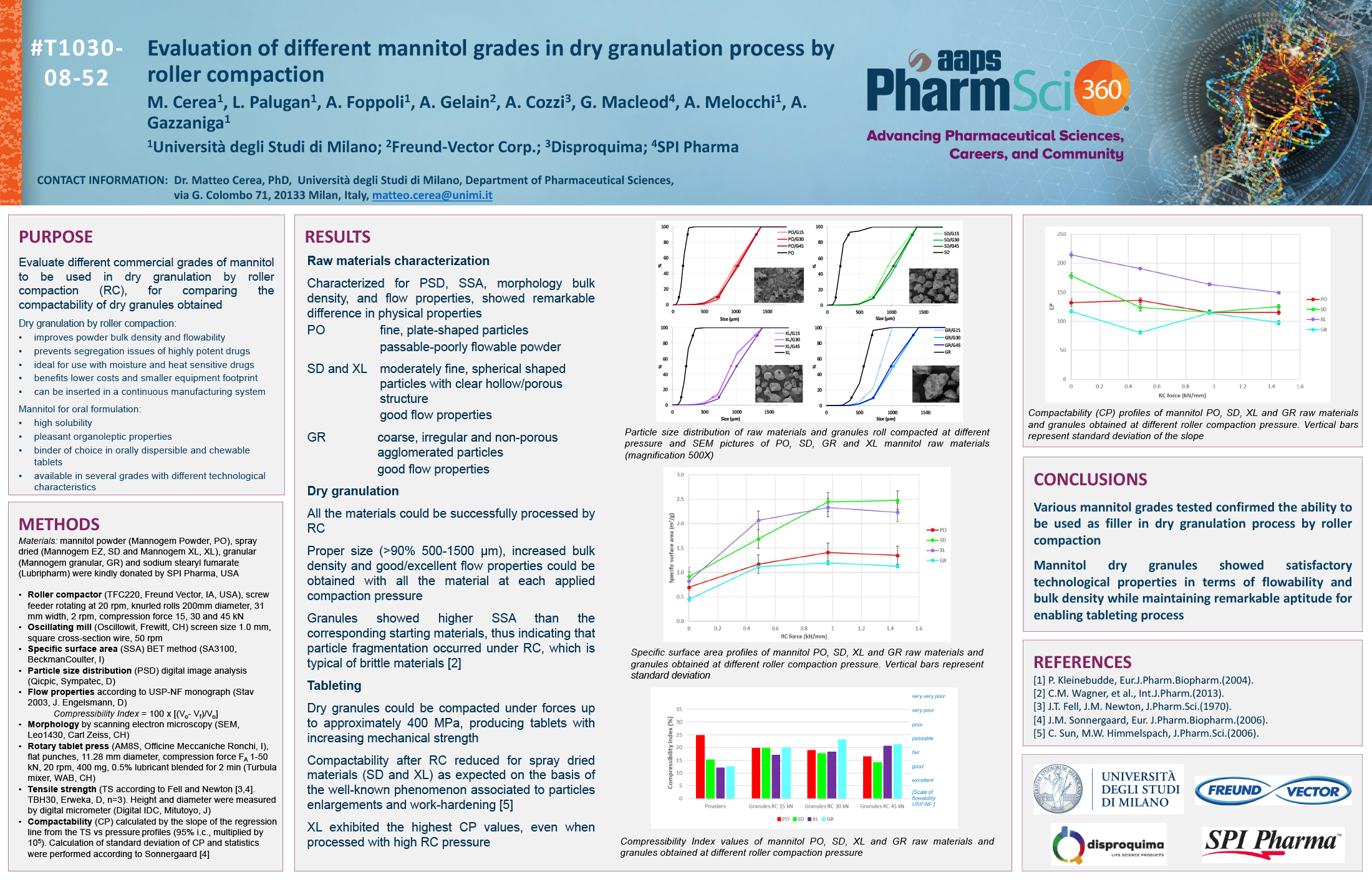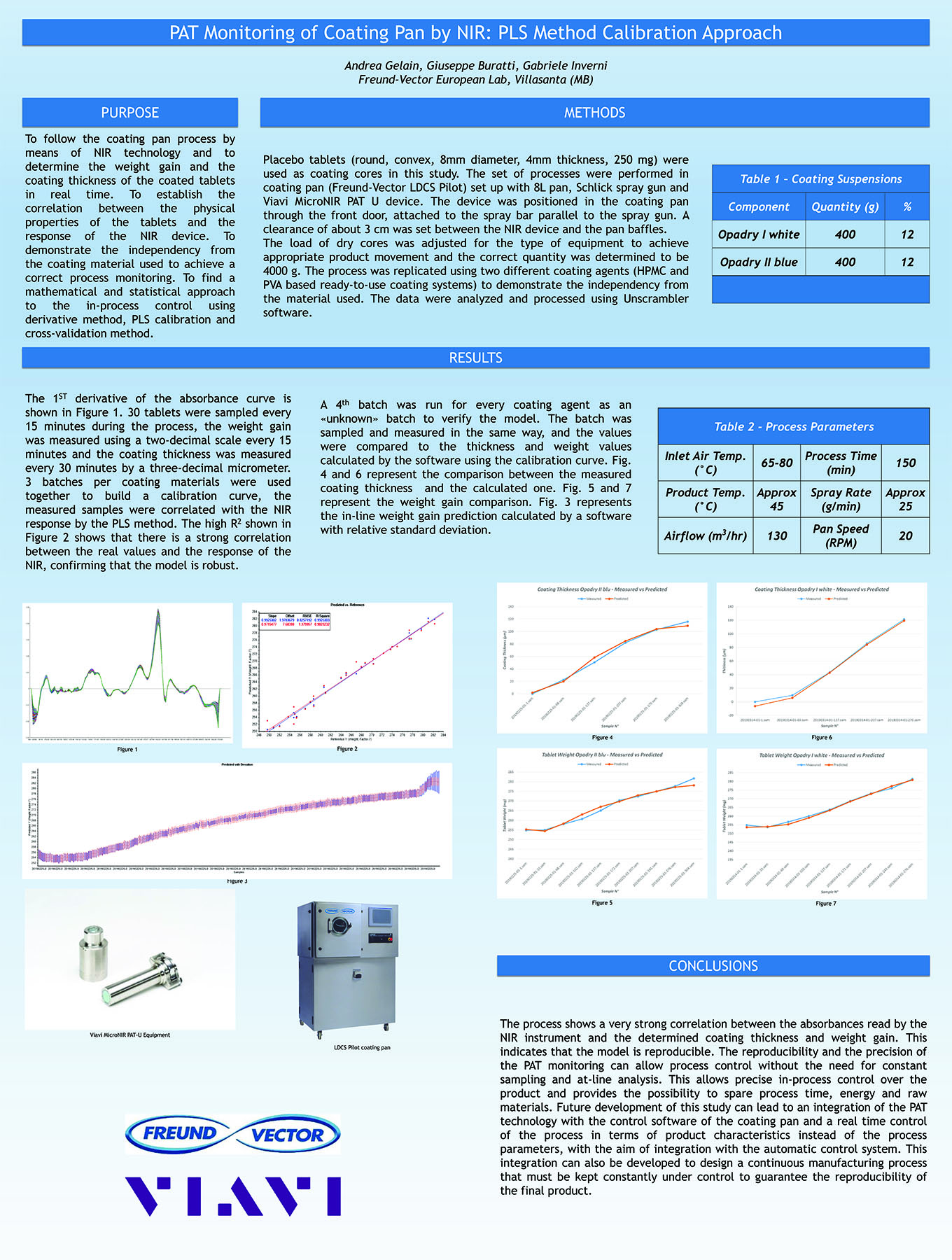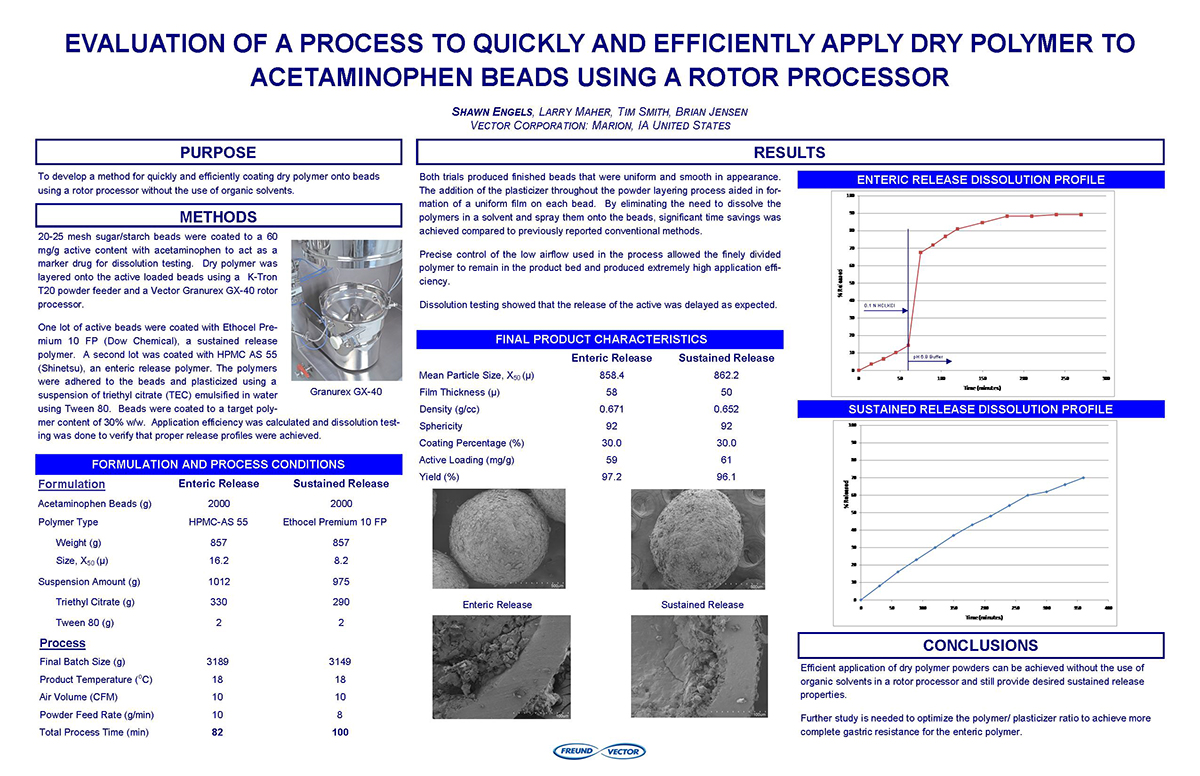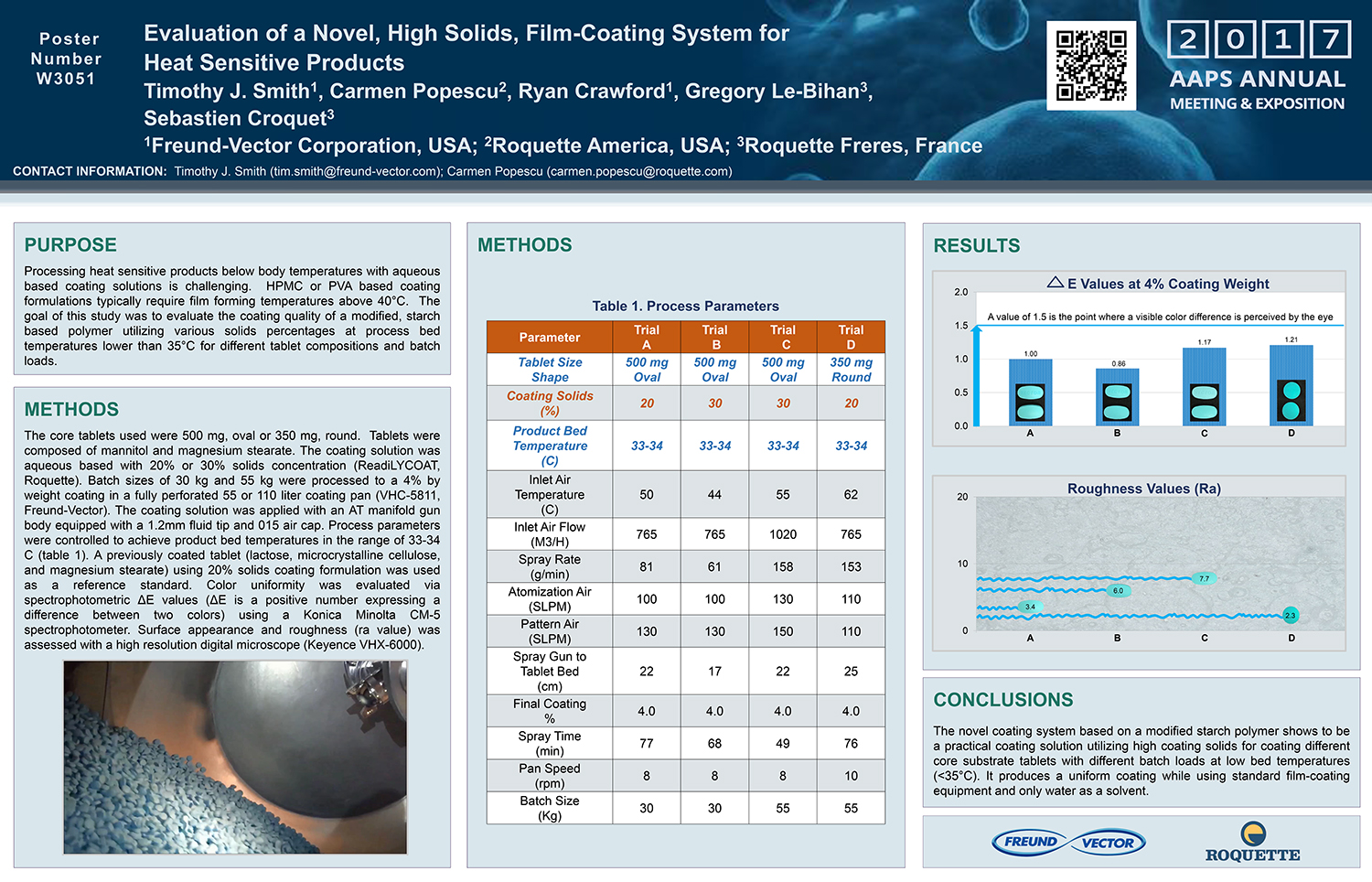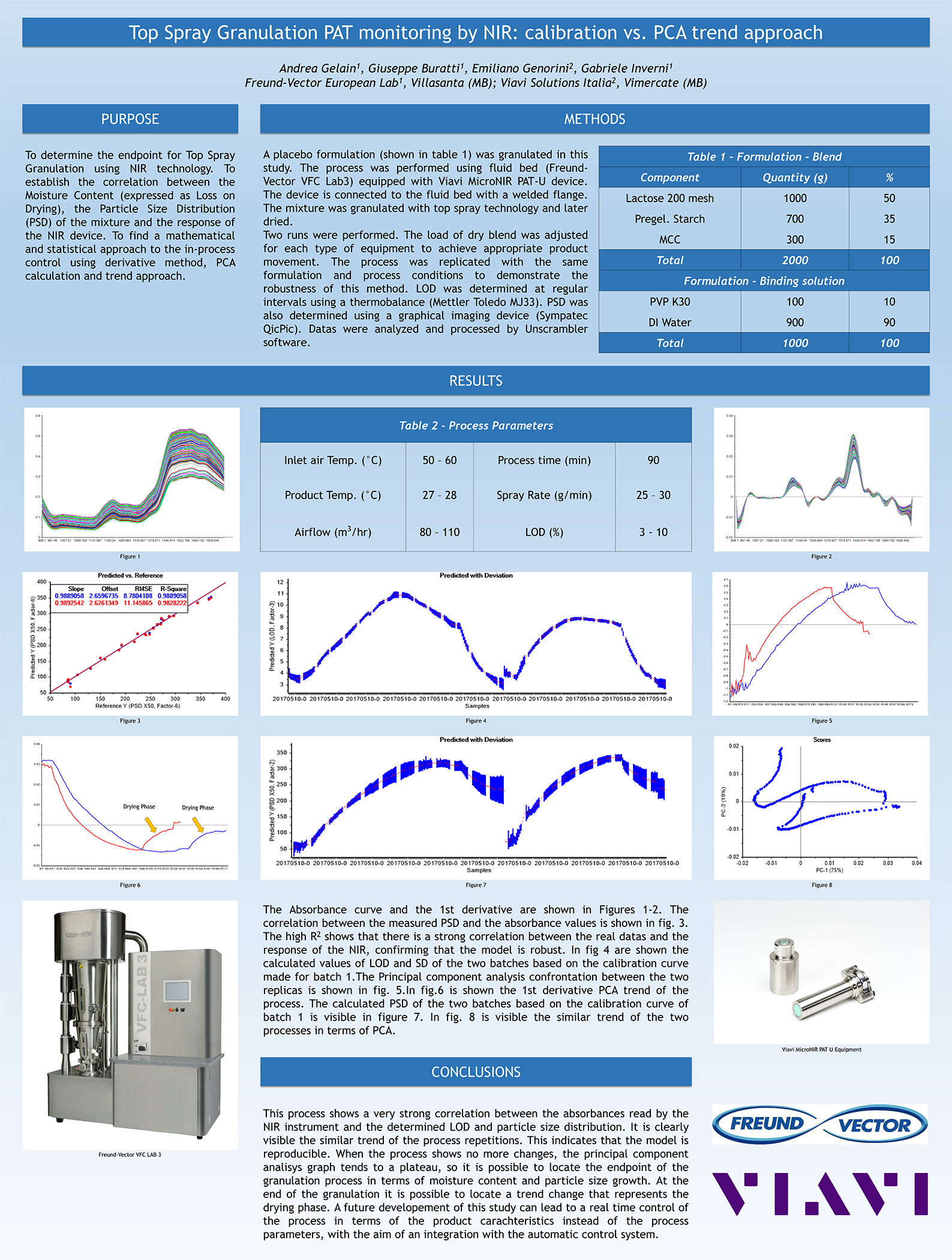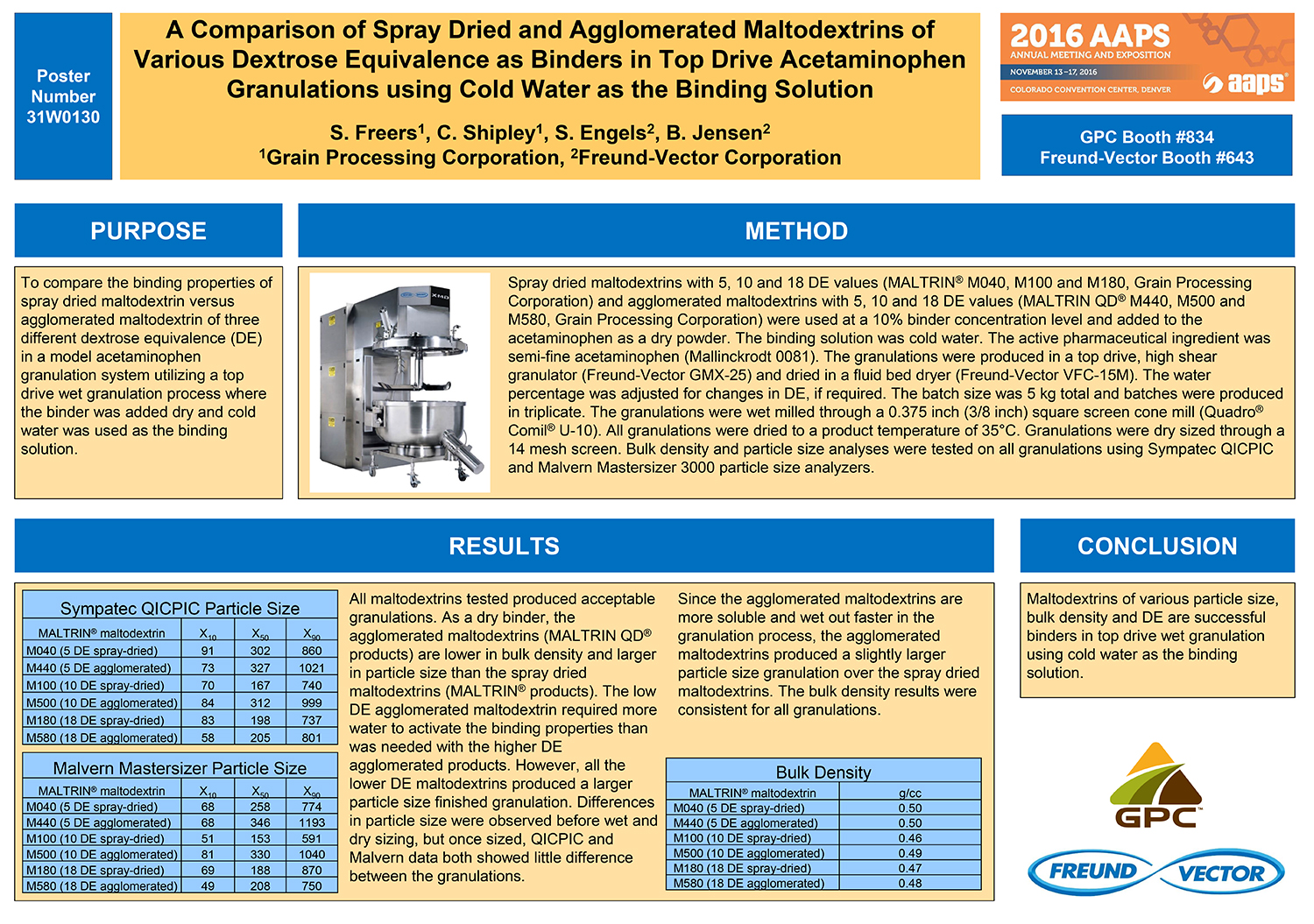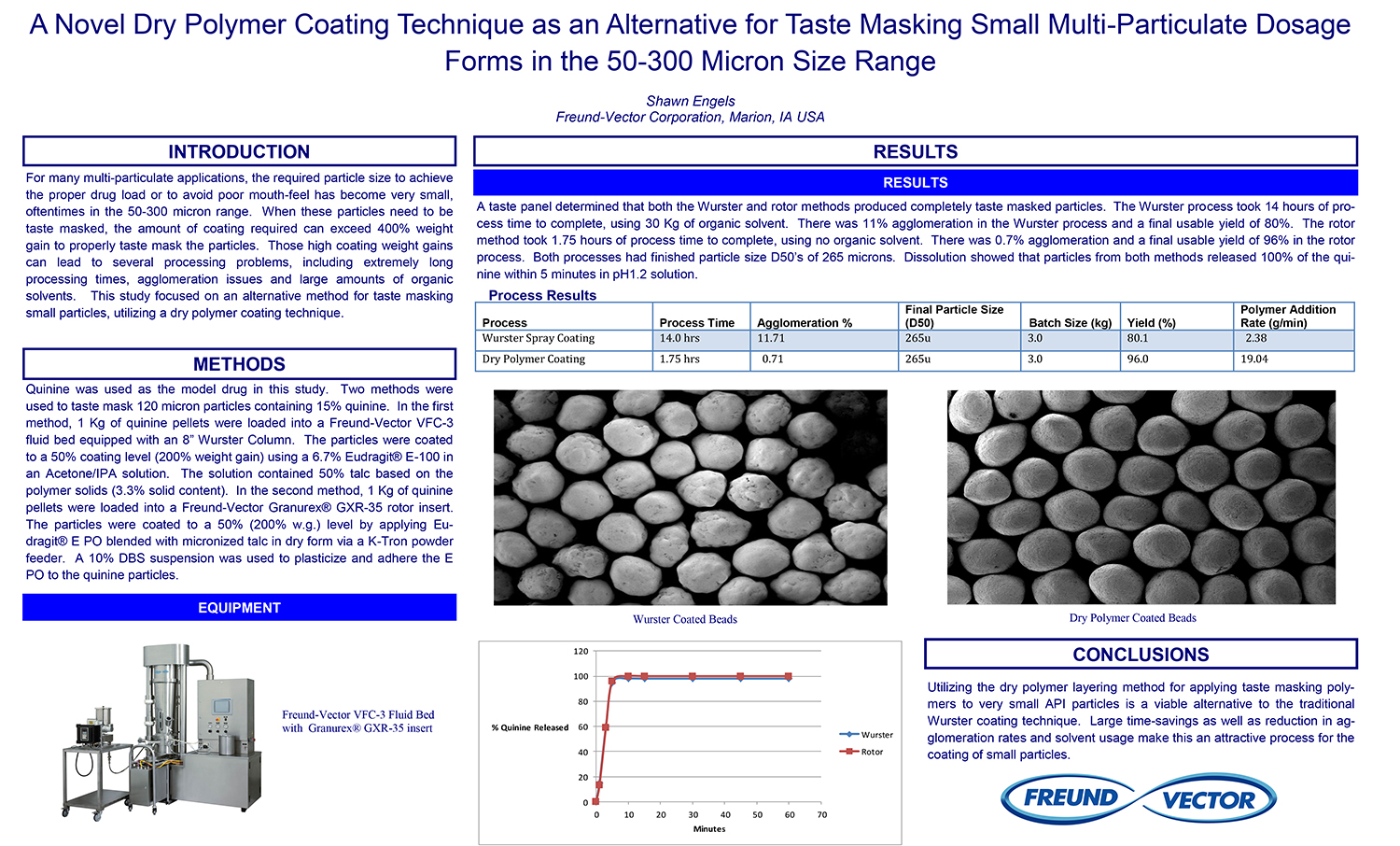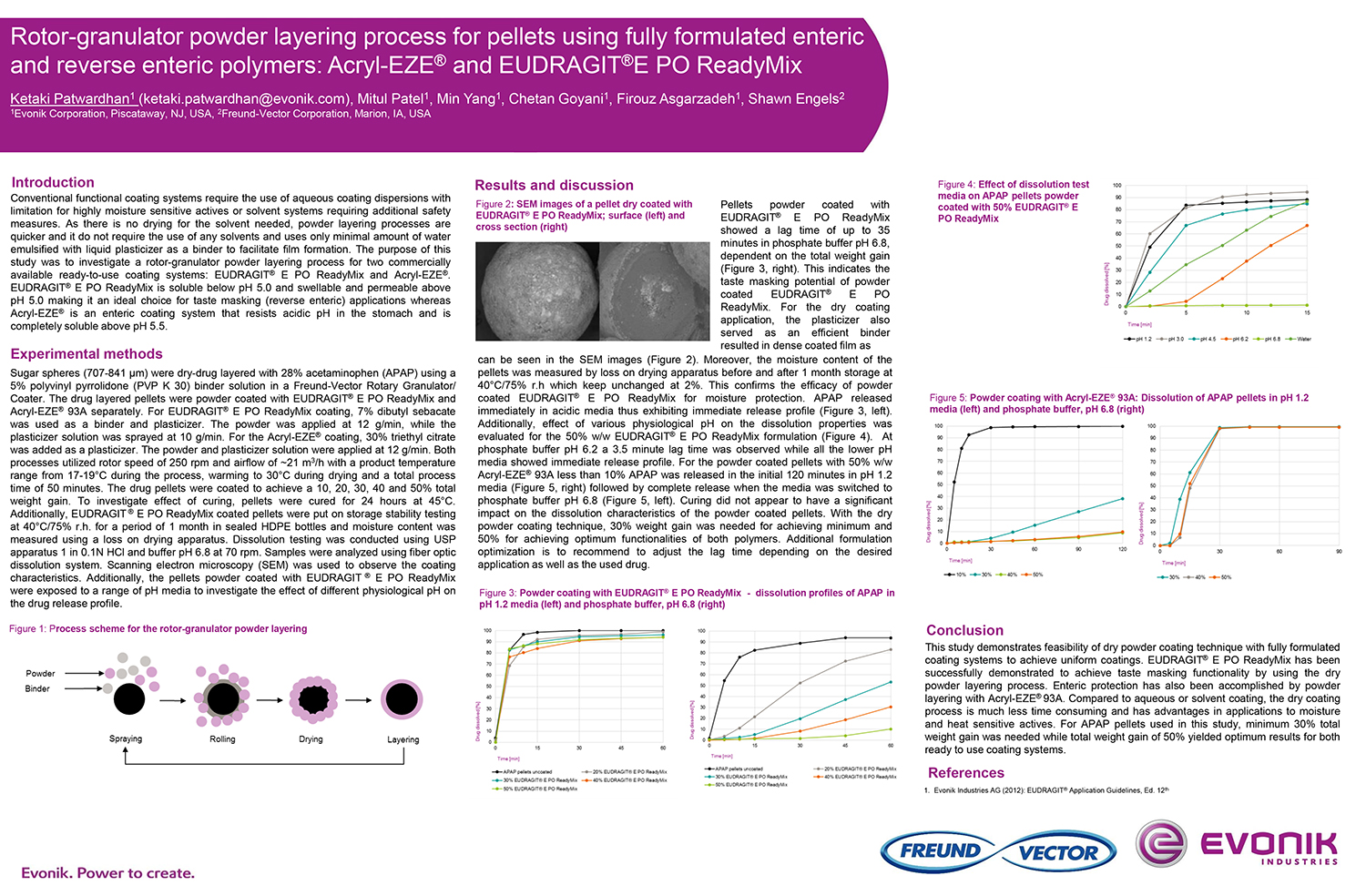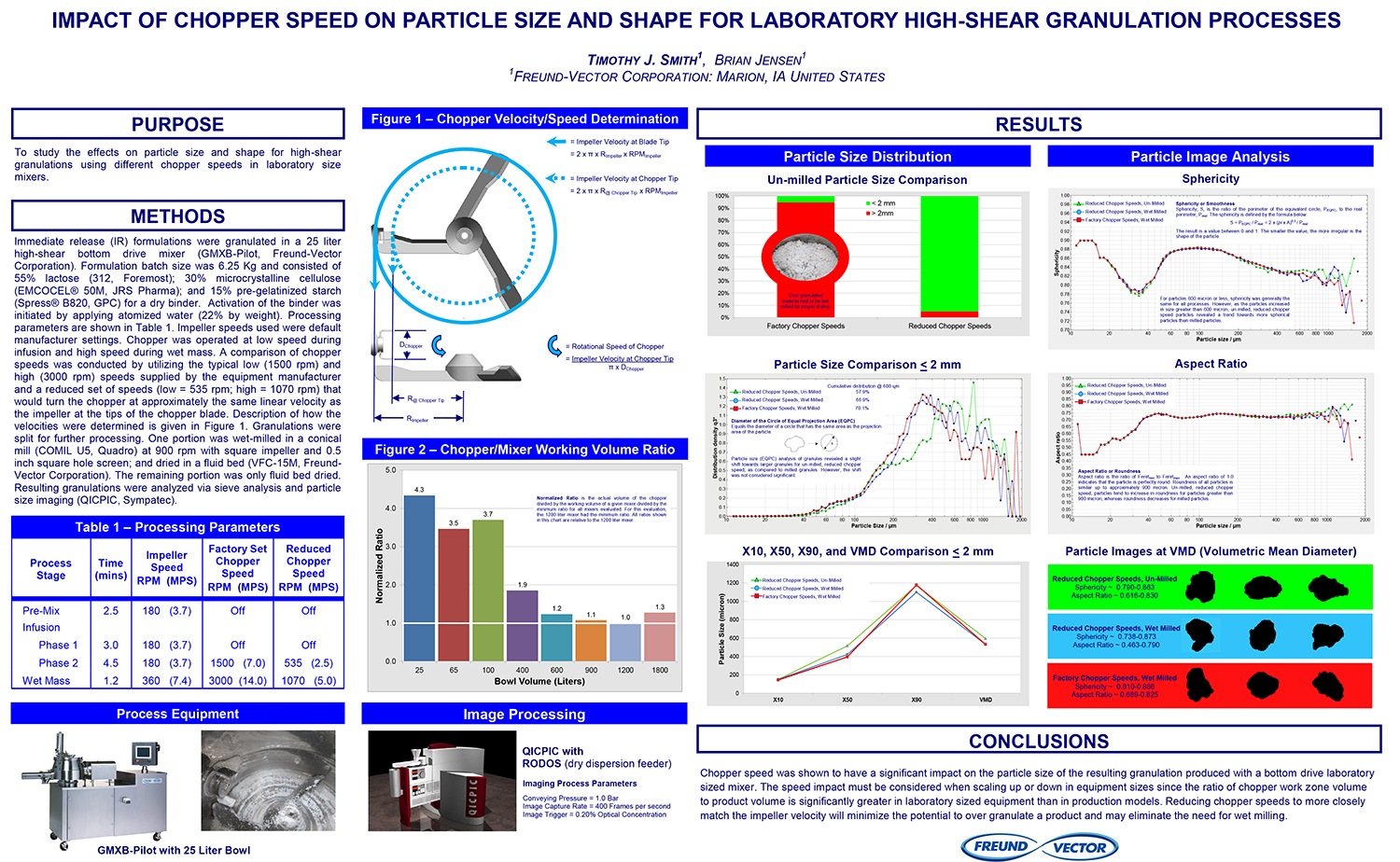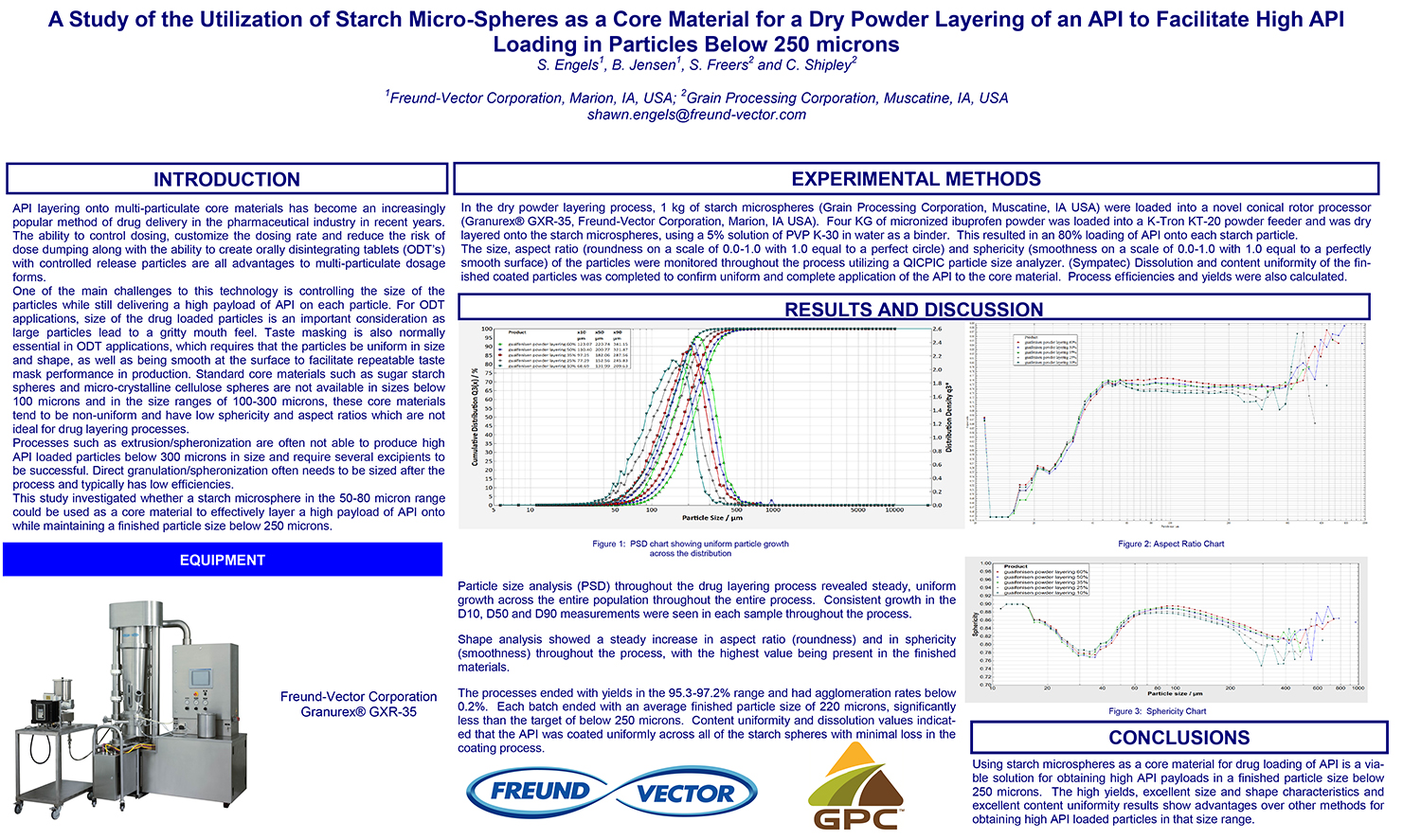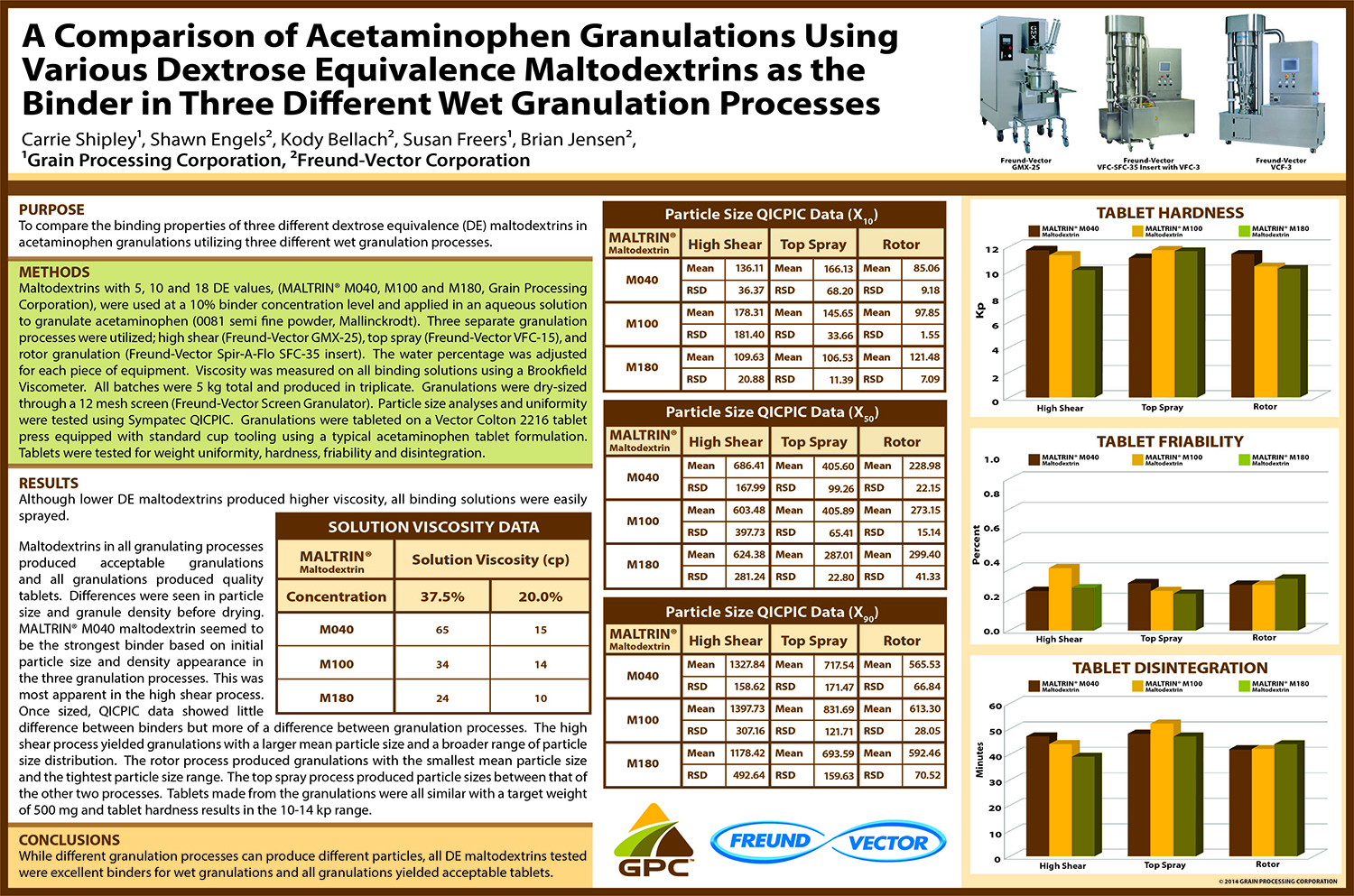
Spray Dry Granulation in Fluid Bed Technology and Scale Up
Granulation can be done using multiple methods such as, high shear mixing, fluid bed granulation, or roll compaction. Wet granulations are typically done in batch processes which can cause bottlenecks in the manufacturing process if spray drying throughputs exceed the batch capabilities of the wet granulation lines, or in cases where granulation capabilities aren’t present, lead to reduced product performance. It is possible to eliminate the need for two machines by combining spray drying and granulation into one singular process. It can also be used to control the final particle size of the material to ensure the desired final product specifications are met. The main purpose of this study was to demonstrate the use of fluid bed technology as a single piece of equipment that is capable of spray drying and granulating in a single process.